RNA splicing factor gene mutations are recurrently found in BCR::ABL1 negative myeloproliferative neoplasms (MPN). To understand the interactions between spliceosome gene mutations and MPN phenotypic driver mutations ( JAK2, CALR and MPL), we investigated their patterns of co-occurrence or mutual exclusivity in a cohort of 990 MPN patients with comprehensive molecular profiling and clinical information from the Hematologic Malignancies Data Repository (HMDR).
As expected, MPN driver mutations ( JAK2, CALR and MPL) were mutually exclusive (N=658, N=168, N=53 respectively, Log2 OR≤-3.5 and Padj. ≤0.005 using pairwise Fisher's exact tests with Benjamin Hochberg multiple testing correction). Additionally, cases with co-occurring mutations in two RNA splicing factors, SF3B1, U2AF1 and SRSF2 (N=44, N=49, N=44) respectively were uncommon (2/135 splicing factor mutated cases) . We further found that U2AF1 and SRSF2 mutations co-occurred with JAK2 (log2 OR=1.7, Padj.=0.009) and MPL (log2 OR=2.1, Padj.=0.006) respectively, but rarely with CALR mutations (N=2 CALR-U2AF1 log2 OR=-2.3 Padj.=0.025, N=2 CALR-SRSF2 log2 OR=-2.2 Padj.=0.048). In contrast, CALR-SF3B1 mutations occurred at the expected frequency (Padj.=0.11).
To explore the molecular settings where these mutations co-occur, we investigated the variant allele frequencies (VAFs) of CALR, U2AF1, SRSF2 and concomitant mutations in the CALR-U2AF1 (N=2) and CALR-SRSF2 (N=2) patients. The VAFs for the mutations detected in the first patient with CALR-U2AF1 co-mutation were: CALR 0.26, U2AF1 0.45, and TET2 0.41. Since CALR and splicing factor mutations are typically heterozygous, this suggests the CALR mutation was sub-clonal to the U2AF1 mutation and that U2AF1 and TET2 mutations were likely present in the same cell. In the second CALR-U2AF1 co-mutant case, VAFs were: CALR 0.31, U2AF1 0.27, and TP53 0.16. It is possible that in this case, the CALR and U2AF1 mutations may have occurred in independent clones or may have occurred in the same cell in the absence of other pathogenic mutations.
For the two CALR-SRSF2 patients, data from three samples revealed VAFs of 0.23-0.46 for CALR and 0.21-0.58 for SRSF2 mutations. Considering their typical heterozygous occurrence, we inferred those individual cells harbored both CALR and SRSF2 mutations. Notably, in samples with high VAFs (>0.37) for both CALR and SRSF2 in two separate patients, additional high VAF pathogenic mutations were observed: 0.35 for ASXL1 and 0.46 for RUNX1 respectively. As ASXL1 and RUNX1 mutations also typically occur in a heterozygous manner, we hypothesized that when CALR and SRSF2 mutations are present in the same cell, another pathogenic co-mutation may be required for survival of the clone.
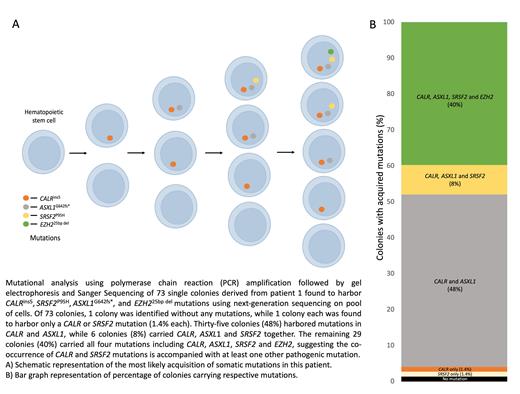
To test our hypothesis, we FACS-sorted single lineage negative CD34 positive bone marrow cells from the first CALR- SRSF2 co-mutant case into 96 wells plates containing methylcellulose and hematopoietic growth factors (CFU-GEMM). DNA was harvested from single-cell colonies and genotyped for the following mutations using polymerase chain reaction (PCR) followed by gel electrophoresis and Sanger sequencing: CALR c.1154_1155insTTGTC p.K385fs*, SRSF2 c.284G>T p.P95H, ASXL1 c.1926_1927insG p.G642fs*, EZH2 c.2188_2212delAAAAAACAGCTCTTCGCCAGTCTGG p.F729fs*. Bulk NGS VAFs were 0.46, 0.38, 0.35, and 0.17, respectively.
Out of 73 colonies analyzed, 71 colonies had the CALR insertion 5 mutation detected (see figure). Among these 71 CALR-mutated colonies, SRSF2 was found to co-occur in 35 colonies. Interestingly, all 35 colonies were accompanied by an ASXL1 mutation, and 29 of them had an additional EZH2 mutation. These findings support our hypothesis that CALR and SRSF2 mutations may co-occur in the same cell in the presence of another pathogenic mutation.
Our findings offer molecular insights into the context-dependent behavior of splicing factor mutations in MPN. While they can co-occur with JAK2 and MPL mutations, U2AF1 and SRSF2 mutations are largely mutually exclusive with CALR mutations. In addition, our findings generate two main biological hypotheses: (i) a synthetic lethal relationship may exist between CALR mutations and SRSF2 or U2AF1 mutations, and (ii) this synthetic lethality may be overcome by the presence of additional pathogenic mutation(s) in the cell. Validation studies are ongoing to address these hypotheses.
AM and AEM contributed equally.
Disclosures
Weeks:Abbvie: Consultancy. Stahl:Kymera: Membership on an entity's Board of Directors or advisory committees; Boston Consulting: Consultancy; Rigel: Membership on an entity's Board of Directors or advisory committees; Clinical care options: Other: GME activity ; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; Haymarket Media: Other: GME activity ; Dedham group: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: GME activity ; GSK: Membership on an entity's Board of Directors or advisory committees; Curis Oncology: Other: GME activity . Luskin:Novartis: Honoraria; Novartis: Research Funding; Pfizer: Honoraria; Jazz: Honoraria; AbbVie: Research Funding. DeAngelo:Amgen, Autolus Therapeutics, Agios, Blueprint, Forty-Seven, Gilead, Incyte, Jazz, Novartis, Pfizer, Servier, Takeda: Consultancy; AbbVie, Glycomimetics, Novartis, Blueprint Pharmaceuticals: Research Funding. Lindsley:Jazz Pharmaceuticals: Consultancy; Vertex Pharmaceuticals: Consultancy; Qiagen: Consultancy; Bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sarepta Therapuetics: Consultancy; Verve Therapuetics: Consultancy; Takeda Pharmaceuticals: Consultancy. Mullally:PharmaEssentia, Incyte: Other: Steering Committee ; Constellation, Protagonist: Other: Advisory Board ; Aclaris, Cellarity, Morphic, Biomarin: Consultancy; AOP Health: Speakers Bureau; Relay, Morphic: Research Funding.